|
About Us
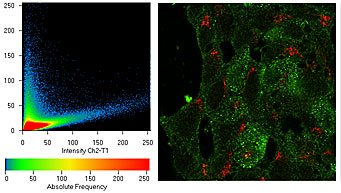
The Michael Hooker Microscopy Facility is a research light microscopy
facility providing advanced digital light microscopy, image processing
and analysis resources to users from the UNC Chapel Hill campus.
We offer instrumentation and instruction to enable users to acquire,
process and analyze images from a wide variety of sample types.



 
 |
Techniques
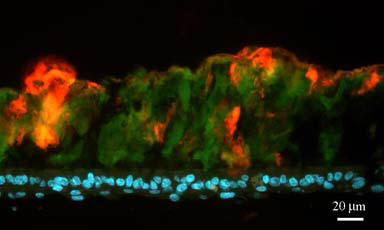
Laser Scanning Confocal
Microscopy (3 systems)
- 4-color fluorescence + DIC
= 5 channel imaging, Visible & UV excitation
- Fluorescence Recovery After
Photobleaching (FRAP) up to 80 fps
- Fluorescence Resonance
Energy Transfer (FRET)
- Fast X-Z scanning
- 3D &/or time lapse image acquisition
- Spectral Imaging – dye
unmixing
- Co-localization
- Time lapse
Live Cell Imaging
- 3-color Spinning-disk
Confocal (FITC, Texas Red & CY5 like dyes)
- Computerized control of
light exposure
- Simultaneous fluorescence and transmitted light (DIC)
- FRET pairs - CFP/YFP, FITC/TRITC,
GFP/mCherry, etc.
- Heated stages
- Temperature and
environmental control
Advanced and Standard
Widefield microscopy
- Ratio imaging on an
inverted microscope, e.g. Ca2+ Fura-2
- Dual camera imaging
- Phase contrast and DIC/Nomarski
- Time-lapse (fluorescence
and transmitted light)
- Intensified CCD camera for
high sensitivity
Laser micro-dissection system
(Leica AS-LMD)
- Isolation of subcellular
and tissue regions for DNA/RNA amplification or mass spectroscopy
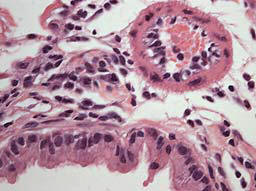
Color brightfield imaging
(high resolution)
- Nikon DXM 1200 pixel shift
color camera (11 MegaPixels)
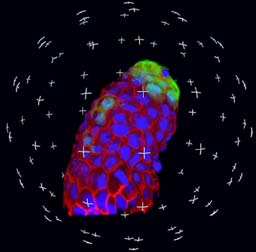
Image Processing and Analysis
- Co-localization
- Particle tracking
- 3-D Image rendering
- Fluorescence quantification
- Densitometry
- Area measurements
- Time lapse analysis
 |
Personnel
Michael Chua
6007 Thurston Bowles
843-3268
microscopy@unc.edu
Neal Kramarcy
6129 Thurston Bowles
966-7051
Equipment
- Confocal:
- Zeiss 510 META Laser
Scanning Confocal with 458, 488, 512, 543, 633 nm lasers
- Leica SP2 AOBS Laser
Scanning Confocal with 4 lasers (351, 364, 458, 488, 514, 561, 633
nm)
- PE Yokogawa Spinning-disk
Confocal with 3-line Kr/Ar laser
- Olympus FV1000 Confocal
with 405, 440, 488, 514, 559, 635 laser lines with Ti:sapphire
multiphoton excitation, motorized x-y stage, box incubator, SIM
scanner.
- Widefield Microscopes:
- Leica DMIRB inverted
microscope with Black and White and color digital cameras; 5X-100X
- Nikon upright microscope
with color digital camera, 2X-100X
- Nikon Elcipse 80i upright
with DIC, phase, fluorescence
- Nikon TE2000 inverted
microscope with ratio imaging, DIC and phase contrast, 2X-100X
- Leica MZ16FA Stereo/macroscope,
motorized z-axis & filter changer
- Environmental Control:
- Bioptechs heated stage
system
- Zeiss Temperature Control
system
- AirTherm air current
incubator
- NuAir 95% humidified 5% CO2
incubator
- Tokai Hit
- Refrigerator (4oC)
- Other:
- Laser micro-dissection
system (Leica)
- Topometrix Explorer Atomic
Force Microscope
- Laser power meter
- Image
Processing/Analysis:
- 4 Windows based image
analysis workstations
- Acquisition and analysis
software packages
- C-Imaging SimplePCI –
measurements, 2D deconvolution
- Metamorph 7 off line
- Volocity – 3D/4D,
quantification, co-localization, tracking - license server for
remote use.
- Zeiss AIM with 3D,
Physiology, time lapse, FRAP, FRET
- Leica LCS software with
FRET, FRAP
- Adobe Creative suite,
Premiere, MS-Office, etc.
- Solid dye color printer
- Flat bed scanner
reflected/transparency
- File server RAID terabyte
networked for
Windows/Mac
- CD/DVD writers, USB,
Firewire connections
 |



